Half of the human population carries Helicobacter pylori, the only known bacterium that survives in the extreme acidic environment inside the stomach. This bacterium damages the mucous coating of the gut after which the stomach acid eats away the sensitive organ lining resulting in peptic ulcers. The infection by this bacterium is also well documented to be a high risk factor of stomach cancer. Despite the effective mean with high efficacy of using antibiotics to battle with this bacterium, claims of antibiotic-resistance over the years have urged the development for new therapies.
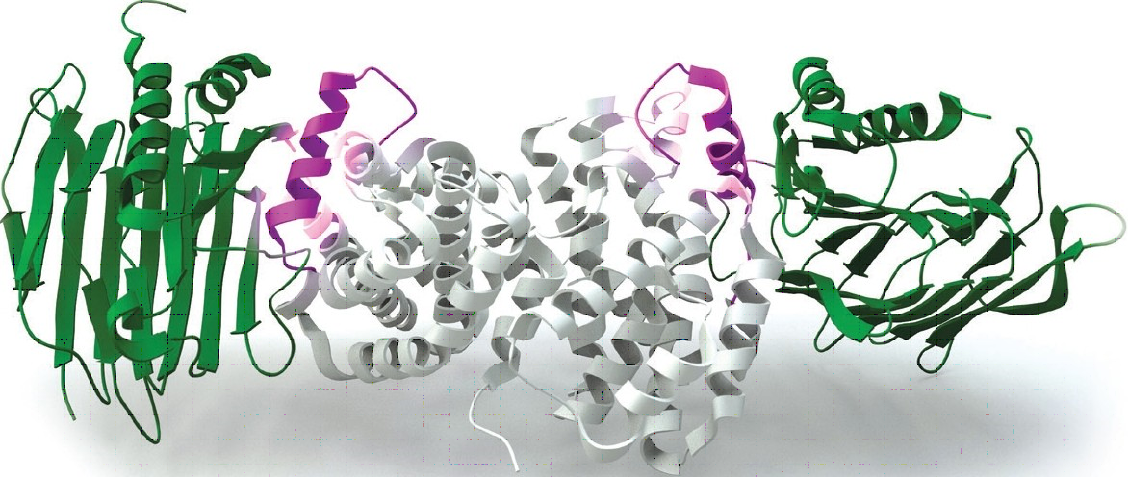
'The key to the survival of Helicobacter pylori in the acidic bath in the human stomach is its use of an enzyme called urease to neutralize gastric acid', said Professor Wong Kam-Bo of the Centre for Protein Science and Crystallography, School of Life Sciences. Urease requires two nickel ions to be functional. Professor Wong and his research team discovered that, using X-ray crystallography to visualize proteins with atomic resolution, the assembly of the urease with the nickel ions involves four helper proteins: UreE, UreF, UreG and UreH. They further revealed that disrupting the formation of the helper protein complex forbids the channeling of the two nickel ions in place and thus inhibits the synthesis of the active urease that is essential to survival.
These conspicuous results have been released lately as 'Paper of the Week' in Journal of Biological Chemistry. Professor Wong said, 'With a better understanding on how the molecular machine is assembled, we can now proceed to study ways that dissemble it. As active urease is the key to the survival of Helicobacter pylori, new drugs designed to target this complex may well be a novel and viable strategy to eradicate the pathogen.' The team is now designing drugs to inhibit assembly of this molecular machine that keeps Helicobacter pylori alive in human stomach.
 |
 |



